Abstract
Background:
DNA hypomethylating agent, decitabine, has become the current standard therapy for patients with higher-risk myelodysplastic syndromes (MDS). Decitabine was launched in China in August 2009 without clinical trials. According to some retrospective studies, the efficacy and safety are similar to those reported in other countries, but there is still a lack of large-scale prospective clinical trials. So we start a prospective clinical trial in China to compare the effect and safety of decitabine in MDS, which was registered at clinicaltrials.gov (NCT02013102).
Design:
Adults with intermediate or high risk MDS by the International Prognostic Scoring System (IPSS≥0.5) were randomized to receive either decitabine 20 mg/m2 IV daily for 5 days (arm Ⅰ) or decitabine 12 mg/m2 IV daily for 8 days (arm Ⅱ) every four weeks. Patients continued to receive study drug for 4 cycles until death, disease progression, intercurrent illness preventing further administration of treatment, unacceptable adverse event or decision by the patient to withdraw from the study. And supportive care were permitted. The primary end point was overall response rate (ORR, CR+mCR+PR) by International Working Group (IWG 2006) criteria, secondary end points included CR, mCR, PR, HI, safety, et al.
Results:
We enrolled a total of 198 patients between 8/2013 and 12/2017, among which 7 patients didn't take decitabine, and 191 were included in the analysis. 94 in arm Ⅰ recieved decitabine and 97 in arm Ⅱ. 32.8% of patients withdrew from the study for a variety of reasons, including progression and death (5.1%), personal decision (13.6%), adverse events (6.6%), and other causes (7.6%).
The median age of patients in arm Ⅰ was 54.88 years old and 54.82 years old in arm II. The median follow-up was 106 days for patients in both arms. The patients received a mean 2.5 cycles of decitabine therapy for arm Ⅰ and 2.0 cycles for arm Ⅱ.
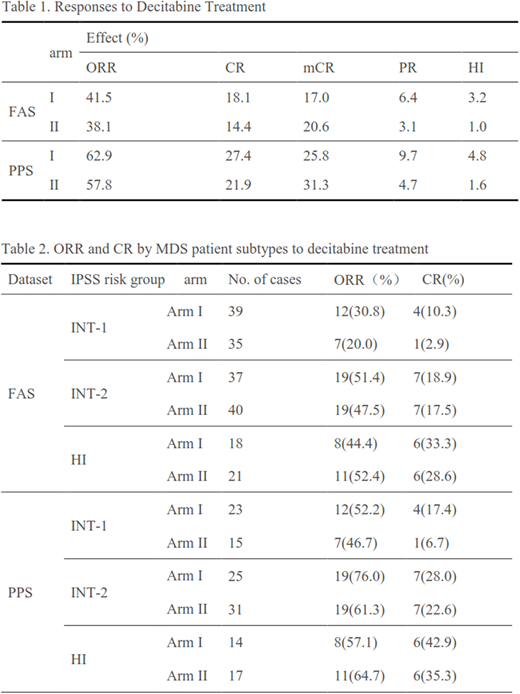
The overall response rate was 39.3% in total, and 41.5% and 38.1% (p=0.6598) for patients in arm Ⅰ and arm Ⅱ, respectively. And CR was 18.1% and 14.4% (p= 0.5584) , PR was 6.4% and 3.1% (p=0.3257) , mCR was 17.0% and 20.6% (p=0.5814) , HI was 3.2% and 1.0% (p=0.3633) , for patients in armⅠand armⅡ, respectively (Table 1). Among all patients, 38.7% were intermediate-1 risk, 40.3% were intermediate-2 risk, 20.4% were high risk. Analysis of response by MDS patient subtypes is shown in Table 2. Those who were higher risk experienced higher ORR and CR, while the difference is not significant between two arms (p>0.05).
As expected, cytopenias were the most frequent complications (76.4%). Grade 3-4 neutropenia, thrombocytopenia and anemia considered to be at least possibly related to the study drug occurred at rates of 23.0%, 34.6%, and 34.6% of patients, respectively. Nonhematologic adverse events were also common including abnormal metabolism and nutrition (23.40% vs 18.56%), abnormal gastrointestinal function (29.79% vs 41.24%), cardiac disorders (11.70% vs 14.43%), infection and infectious diseases (32.98% vs 36.08%), abnormal skin and subcutaneous tissue and so on, which were no significant differences between two ams. During the study there were 17 SAE, only 7 cases were possibly related to drug therapy, such as pulmonary infection, Sepsis, myelosuppression, intracranial hemorrhage, hepatic failure, and arrhythmia.
Conclusions:
The use of 5-day and 8-day schedule decitabine is safe and effective in patients with intermediate and high risk MDS, among which there was no significant differences.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal